.png)
By Diya Asawa, 2024
Using Electrospinning to Develop Fast-Dissolving Medicines
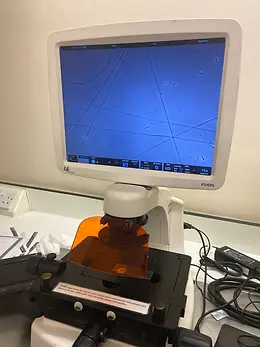
Over the summer of 2024, I had the incredible opportunity to work at a lab in the UCL School of Pharmacy under the mentorship of Dr Karolina Dziemidowicz (Professor of Pharmaceutics) and Dr Catherine Tuleu (Professor of Paediatric Pharmaceutics). With the guidance and support of my supervisors and a team of postgraduate students, I learnt how to use electrospinning equipment (a nanofibre-production method) to develop placebo orodispersible films (ODFs), gaining insights into drug delivery, discovery, and development (see figures 1 & 2).
Figures 1 & 2: Images of the electrospinning equipment (left) used to create thin placebo films (right). The placebo formulation consisted of a polymer solution combined with rhodamine, an artificial dye.
Orodispersible Films
The last time you browsed the shelves of your local pharmacy, you may have noticed that medicines can take the shape of a variety of dosage forms, including tablets, capsules, liquids, ointments, and gels (the list goes on). An orodispersible film (ODF) is a relatively novel dosage form designed to quickly disintegrate on the tongue and then be absorbed by the mucous membranes within the oral cavity. These films, which look like small postage stamps, are thin polymeric sheets made from either single or multiple layers of electrospun fibres.
Upon contact with saliva, ODFs undergo rapid disintegration without the need for additional water, which can be useful in locations with limited access to clean water. Additionally, ODFs may be suitable alternatives for young children and elderly patients who often struggle with taking traditional medicines. For example, tablets are usually quite large and contain inert fillers like calcium carbonate, which makes them bitter and unpalatable. Other common dosage forms like liquids may contain added sugar which can cause dental problems, while administration methods like injections are painful and require hospital admission, placing a strain on hospital resources.
Researchers at the UCL School of Pharmacy are currently trying to load antiemetic drugs into ODFs (to treat nausea and sickness caused by cancer chemotherapy). Using a polymer solution containing the active pharmaceutical ingredient and several inactive substances or excipients, researchers can use electrospinning equipment to “spin” the drug formulation, creating polymeric sheets of fibres which are then cut into appropriate sizes to form ODFs.
Electrospinning
The process of electrospinning (see figure 3) to produce ODFs is similar to spinning liquid sugar to create candy floss. However, rather than liquid sugar and heat, a polymer solution and electricity are used instead. The polymer solution is pumped out of a syringe at a specified rate and charged with a high voltage. This solution is then forced out of a spinneret (a metal nozzle) to form a jet of charged particles known as the Taylor cone.
Figure 3: A diagram explaining the set-up of an electrospinning machine. A polymer solution and a high voltage power supply are used to create polymeric fibres. (Image credit: Rana et al., 2017).
Under the right conditions, a circular, whipping motion of the charged particles leads to the formation of thread-like fibres on the machine’s metal collector. As the polymer cools down and the solvent fully evaporates, solid fibres develop on the metal collector. Electrospinning is therefore a highly scalable and cost-effective technique that can be used to mass-produce ODFs in the pharmaceutical industry (see figure 4). Interestingly, the active pharmaceutical ingredients in ODFs are not only limited to conventional drugs; they can also include substances found in vaccines, probiotics, plant-based products, and vitamins.
Figure 4: An image of an electrospinning machine (with a cylindrical metal collector) at the UCL School of Pharmacy which can be used for large-scale production of ODFs.
Formulation Optimisation
To prepare the drug formulation, a polymer solution is prepared using a polymer and a solvent. A biodegradable polymer is used to form the main structure or matrix of the ODF, creating a continuous film that forms once the solvent evaporates during electrospinning. Since polymers on their own usually form rigid and brittle structures, the addition of plasticisers improves their overall flexibility and strength. An organic, volatile solvent is then added to the mixture. High volatility allows the rapid evaporation of the solvent during electrospinning, forming a dry polymeric film. Afterwards, the polymer solution is heated up and stirred using a magnetic stirrer to develop a homogeneous solution.
To create the drug formulation, the active pharmaceutical ingredient or drug is then incorporated into the mixture and fully dissolved. Additional excipients (inert substances) such as taste-masking agents, preservatives, and disintegrants are also added to the formulation to modify properties like flavour, stability, and dissolution rate.
During my time at the UCL School of Pharmacy, I learnt how to optimise a placebo formulation for electrospinning, a process requiring the modification of various parameters. An important parameter that I had to consider was how hydrophobic (water-fearing) or hydrophilic (water-loving) the polymer and solvent were, since “like dissolves like.” For example, ethanol has some nonpolar character due to the presence of an ethyl group (-CH2CH3). This causes the polymer polyvinyl alcohol (PVA), which is highly hydrophilic, to display lower solubility in ethanol compared to water. Therefore, PVA had to be dissolved in a mixture containing both ethanol and water.
Another vital parameter for electrospinning is the viscosity of the formulation. A sufficient level of viscosity combined with the right voltage can help overcome the surface tension forces which usually maintain the liquid’s droplet shape. For example, for my first formulation, I used 2 grams of 12% PVA (of low molecular weight) in a 20 ml solution (50% ethanol and 50% water). However, upon viewing the electrospun sample under the light microscope, I noticed that it ended up forming droplets/particles rather than fibres, suggesting that the solution had low viscosity. After creating various unsuccessful formulations, I finally used 4 grams of 20% PVA (of higher molecular weight) in the same 20 ml solution (50% ethanol and 50% water), which proved successful for forming PVA fibres (see figure 5). Therefore, through trial-and-error, I learnt that increasing the molecular weight and concentration of the added polymer can modify the viscosity of the formulation.
Figure 5: An image of the PVA fibres observed using a light microscope.
Other key parameters include flow rate, humidity, voltage, and the distance between the capillary needle and the metal collector. For instance, a higher flow rate indicates that a greater volume of fluid is being forced through the spinneret within a given time period, causing the formation of thicker diameter fibres. However, a flow rate that is too high will cause the Taylor cone (the jet of polymer solution) to become unstable due to the impact of gravitational forces, which can cause droplets or particles to form instead of fibres. Additionally, I learnt that low relative humidity in the lab environment was optimal because it allowed the solvent to evaporate more quickly into the air during electrospinning, aiding the formation of dry, consistent fibres. Increasing the voltage and decreasing the distance between the capillary needle and collector created a stronger electric field, which was vital for overcoming the surface tension of the liquid formulation and forming consistent fibres.
Drug Delivery
ODFs typically disintegrate within seconds in the mouth, forming a suspension or a solution which allows for quicker absorption and drug delivery into the bloodstream, demonstrating rapid local effects. The oral cavity is lined with many blood vessels and relatively low enzymatic action, which makes it easier for drugs to permeate through the mucous membranes. Additionally, quick permeation through the oral cavity helps the drug evade acidic hydrolysis in the stomach or chemical modifications in the liver, which increases the drug’s bioavailability (the amount of drug that reaches the target biological site). However, ODFs can be engineered to suit different forms of drug delivery, allowing for a wide range of medical applications.
Similar to how clothes are packed inside a suitcase, drugs can be encapsulated by a protective layer or covering. Encapsulation can be achieved via coaxial electrospinning, which involves the use of 2 syringes with different solutions or components. The components of these syringes are simultaneously fed into different capillary needles, allowing for the formation of fibres with a core layer and an outer shell layer. The core of these fibres usually contains the bioactive drug while the protective shell consists of biodegradable polymers like polycaprolactone (PCL) and other materials that provide structural strength (see figure 6).
Figure 6: A rough sketch illustrating the difference between a monoaxial and a coaxial fibre created using electrospinning (inspiration for this diagram: Kellaway et al., 2024).
The properties of the protective shell (such as its porosity, thickness, and mechanism of binding to the drug) can be modified, allowing for the controlled release of the drug over a planned time frame as opposed to all at once. Coaxial electrospinning not only leads to higher loading efficiency of the drug into the ODF, but also prevents the drug from being degraded by enzymes or harsh conditions before it reaches its target site in the body.
Interestingly, multifunctional drug delivery using ODFs could help pave the path for combination therapies and targeted drug delivery. While most ODFs currently contain a single drug, researchers have recently tried to load multiple drugs into ODFs, which could be useful for combination therapies. For example, in a study done by Javed et al. (2022), researchers prepared ODFs containing 2 drugs known to treat migraine and associated nausea & vomiting. Fibres produced by electrospinning can also be loaded with nanoparticles to allow for more precise drug delivery to the target region of the body. Additionally, the use of nanoparticles could make it easier to transport water-insoluble drugs via the body’s blood circulation.
From Lab to Market
Before medicines are approved by regulatory bodies like the FDA, various factors such as safety and efficiency need to be considered throughout the drug development process. For example, it is important to consider the toxicity of solvents and assess how flammable or carcinogenic they may be. Drugs with low biocompatibility may cause adverse immune reactions in patients, while drugs with low biodegradability could potentially cause a build-up of toxic waste products in the body. Being efficient with allocated funding and resources can help minimise the waste of lab materials and reduce the costs of obtaining, handling, or disposing off chemicals.
Over the course of the summer, I gained insights into various factors that researchers must consider before attempting to commercialise scientific technologies. For instance, in paediatric medicine, healthcare governing boards require research evidence that children will be willing and able to take novel dosage forms like ODFs, since this provides incentive for new pharmaceutical research to be funded and translated from lab to market. After spending days combing through research papers related to this research area, I learnt that there is currently a lack of sufficient literature on the acceptability of ODFs in paediatric populations (within the UK) since ODFs are relatively novel dosage forms.
One of the main studies done in this research area (Orlu et al, 2017) illustrated that orally administrated liquids had some of the highest preference ratings; however, ODFs also received positive acceptability from young children and infants despite a lack of prior experience with taking the dosage form. Interestingly, ODFs could be engineered to deliver medicines via transdermal or ocular routes as well, making drug administration easier for young children with selective taste preferences. Increased familiarity with ODFs and their potential administration routes could therefore help change public perception on dosage form preferences and make patients more willing to try new types of drug formulations.
References
Ahmadi Bonakdar, M. and Rodrigue, D. (2024). Electrospinning: Processes, Structures, and Materials. Macromol, [online] 4(1), pp.58–103. doi:https://doi.org/10.3390/macromol4010004.
Cupone, I.E., Sansone, A., Marra, F., Giori, A.M. and Jannini, E.A. (2022). Orodispersible Film (ODF) Platform Based on Maltodextrin for Therapeutical Applications. Pharmaceutics, 14(10), p.2011. doi:https://doi.org/10.3390/pharmaceutics14102011. Ferlak, J., Guzenda, W. and Osmałek, T. (2023).
Orodispersible Films—Current State of the Art, Limitations, Advances and Future Perspectives. Pharmaceutics, 15(2), p.361. doi:https://doi.org/10.3390/pharmaceutics15020361. Jacob, S., Boddu, S.H.S., Bhandare, R., Ahmad, S.S. and Nair, A.B. (2023). Orodispersible Films: Current Innovations and Emerging Trends. Pharmaceutics, [online] 15(12), p.2753. doi:https://doi.org/10.3390/pharmaceutics15122753. Javed, S., Hussain, A., Shah, P.A., Ishtiaq, S.,
Ali, E., Abbas, N. and Bukhari, N.I. (2022). Simultaneous quantification of sumatriptan succinate and prochlorperazine maleate in orodispersible films using two validated UV-spectroscopic methods. | Pakistan Journal of Pharmaceutical Sciences | EBSCOhost. Ebsco.com, [online] 35, p.183. doi:https://doi.org/10.36721/PJPS.2022.35.1.SUP.183-194.1.
Johns Hopkins Medicine (2023). Liver: Anatomy and Functions. [online] Johns Hopkins Medicine. Available at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/liver-anatomy-and-functions.
Kellaway, S.C., Ullrich, M.M. and Karolina Dziemidowicz (2024). Electrospun drug‐loaded scaffolds for nervous system repair. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology, 16(3). doi:https://doi.org/10.1002/wnan.1965.
Martínez-Pérez, C.A. (2020). Electrospinning: A promising technique for drug delivery systems. REVIEWS ON ADVANCED MATERIALS SCIENCE, 59(1), pp.441–454. doi:https://doi.org/10.1515/rams-2020-0041.
Nureddin Ashammakhi, Tavafoghi, M., Jafari, A., Sumama Nuthana Kalva, Augustine, R., Hasan, A., Houman Savoji, Yavuz Nuri Ertas and Li, S. (2022). Electrospinning and Three-Dimensional (3D) Printing for Biofabrication. Springer eBooks, pp.555–604. doi:https://doi.org/10.1007/978-3-030-99958-2_20.
Orlu, M., Ranmal, S.R., Sheng, Y., Tuleu, C. and Seddon, P. (2017). Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Delivery, 24(1), pp.1243–1248. doi:https://doi.org/10.1080/10717544.2017.1370512.
Pant, B., Park, M. and Park, S.-J. (2019). Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics, 11(7), p.305. doi:https://doi.org/10.3390/pharmaceutics11070305.
Pünnel, L.C. and Lunter, D.J. (2021). Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics, 13(7), p.932. doi:https://doi.org/10.3390/pharmaceutics13070932. Qin, X. (2017). 3 - Coaxial electrospinning of nanofibers. [online] ScienceDirect. Available at: https://www.sciencedirect.com/science/article/abs/pii/B9780081009079000039. Raksa, A.,
Numpaisal, P. and Ruksakulpiwat, Y. (2021). The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Materials Today: Proceedings, 47, pp.3458–3461. doi:https://doi.org/10.1016/j.matpr.2021.03.459.
Rana, D., Ramasamy, K., Samad Ahadian, Geetha Manivasagam, Wang, X. and Ramalingam, M. (2017). Polymeric Nanobiomaterials. pp.65–84. doi:https://doi.org/10.1002/9783527698646.ch3.
Rand Abdulhussain, Adeola Adebisi, Conway, B.R. and Kofi Asare-Addo (2023). Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. Journal of Drug Delivery Science and Technology, 90, pp.105156–105156. doi:https://doi.org/10.1016/j.jddst.2023.105156.
Savjani, K.T., Gajjar, A.K. and Savjani, J.K. (2012). Drug solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics, 2012, pp.1–10. doi:https://doi.org/10.5402/2012/195727.
Scarpa, M., Stegemann, S., Hsiao, W.-K., Pichler, H., Gaisford, S., Bresciani, M., Paudel, A. and Orlu, M. (n.d.). Orodispersible Films: Towards Drug Delivery in Special Populations. [online] Available at: https://discovery.ucl.ac.uk/id/eprint/1547416/1/Orlu-M_orodispersible%20films_drug%20delive ry_.pdf [Accessed 25 Sep. 2024].
Sharma, G.K. and James, N.R. (2022). Electrospinning: The Technique and Applications. [online] www.intechopen.com. Available at: https://www.intechopen.com/chapters/83223. Spinneret | Synthetic Fibres, Polymerization & Spinning | Britannica. (2024). In: Encyclopædia Britannica. [online] Available at: https://www.britannica.com/technology/spinneret-fibre-manufacturing#:~:text=Spinneret%2C% 20in%20the%20spinning%20of%20man-made%20fibre%2C%20small%2C [Accessed 25 Sep. 2024].
Suresh, S., Becker, A. and Glasmacher, B. (2020). Impact of Apparatus Orientation and Gravity in Electrospinning-A Review of Empirical Evidence. Polymers, [online] 12(11). doi:https://doi.org/10.3390/polym12112448.
Tian, Y., Lin, J., Jing, H., Wang, Q., Wu, Z. and Duan, Y. (2023). Recent progress in orodispersible films‐mediated therapeutic applications: A review. MedComm - Biomaterials and applications, 2(2). doi:https://doi.org/10.1002/mba2.34.
Xue, J., Wu, T., Dai, Y. and Xia, Y. (2019). Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chemical Reviews, [online] 119(8), pp.5298–5415. doi:https://doi.org/10.1021/acs.chemrev.8b00593.
Zhang, H., Jin, C., Shihao Lv, Ren, F. and Wang, J. (2023). Study on electrospinning of wheat gluten: A review. Food Research International, 169, pp.112851–112851. doi:https://doi.org/10.1016/j.foodres.2023.112851.